Molecular Strep A Test Cleared for Cobas System
By LabMedica International staff writers
Posted on 03 Dec 2014
A rapid test for the detection of group A streptococcus bacterial (Strep A) DNA in throat swab specimens has been cleared for use in a molecular point of care diagnostic system.Posted on 03 Dec 2014
The Strep A test achieved outstanding sensitivity aiding healthcare professionals to make immediate, informed treatment decisions in a variety of testing locations and accurate diagnosis of acute infection is necessary to properly treat the disease using appropriate antibiotic therapy.

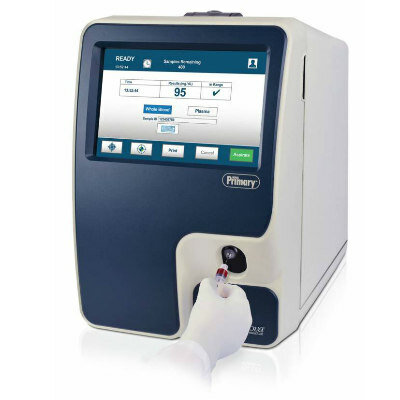
Image: The cobas Liat molecular diagnostic system (Photo courtesy of Roche).
Streptococcus pyogenes (Strep A) is a ubiquitous pathogen that causes a wide range of human infections, including pharyngitis, sinusitis, lymphadenitis, pyoderma, endocarditis, meningitis, septicemia, tonsillitis, impetigo, and upper respiratory tract infections. Strep A is capable of initiating two nonsuppurative complications, acute rheumatic fever and post-streptococcal acute glomerulonephritis, which can have severe negative consequences on the health and well-being of infected patients.
The cobas Strep A assay utilizes polymerase chain reaction (PCR) technology and the cobas Strep A test can detect Strep A DNA obtained from throat swab specimens in 15 minutes with the cobas Liat System (Roche; Basel, Switzerland). The cobas Strep A test is Conformité Européenne (CE) Marked and the US Food and Drug Administration (FDA; Silver Spring, MD, USA) has provided 510(k) clearance. The cobas Liat is a compact, fast and easy to use molecular diagnostic system designed for on-demand testing in physician clinics, pharmacy, and hospital laboratory settings. The system includes the cobas Liat Analyzer and growing portfolio of assays, including cobas Influenza A/B and cobas Strep A.
Roland Diggelmann, MBA, Chief Operating Officer of Roche Diagnostics, said, “The cobas Strep A test is easy to use and provides accurate results to support a treatment decision in just 15 minutes, much faster than current technologies. It also provides a significant improvement over conventional methods such as culture testing, where patients can wait up to two days to receive their result, or rapid antigen testing where confirmation with culture is needed due to significantly lower sensitivity.”
Related Links:
Roche
US Food and Drug Administration